Welcome to COMBINE-CT!
We are excited to present our project. We are a consortium of clinical centres, research institutes, and healthcare companies, with the goal of bringing the CT scan to the forefront of the diagnosis, treatment planning, and follow-up of patients with coronary artery disease.
In this newsletter, we will:
- Give an overview of the problem we want to solve and state our goals
- Present the clinical studies that will be carried out during the project
- Show a short video answering how patients will benefit from the COMBINE-CT project
- Introduce two of the partners of the consortium, IBSAL & CIBER

Overview of the Project
Coronary artery disease is the most common heart condition, and the leading cause of death globally. It consists in the narrowing of cardiac arteries due to the build-up of atherosclerotic plaque, caused by high cholesterol and other risk factors. This, in turn, causes a decrease in the blood flow that reaches the heart muscle, which can cause myocardial infarction, that is to say, the death of the muscle tissue of the heart. This is a life-threatening situation that usually manifests as chest pain. Treatment consists of a catheter guided intervention, in which the narrowed arteries are opened, and a small device called stent is implanted to keep these arteries from narrowing again.
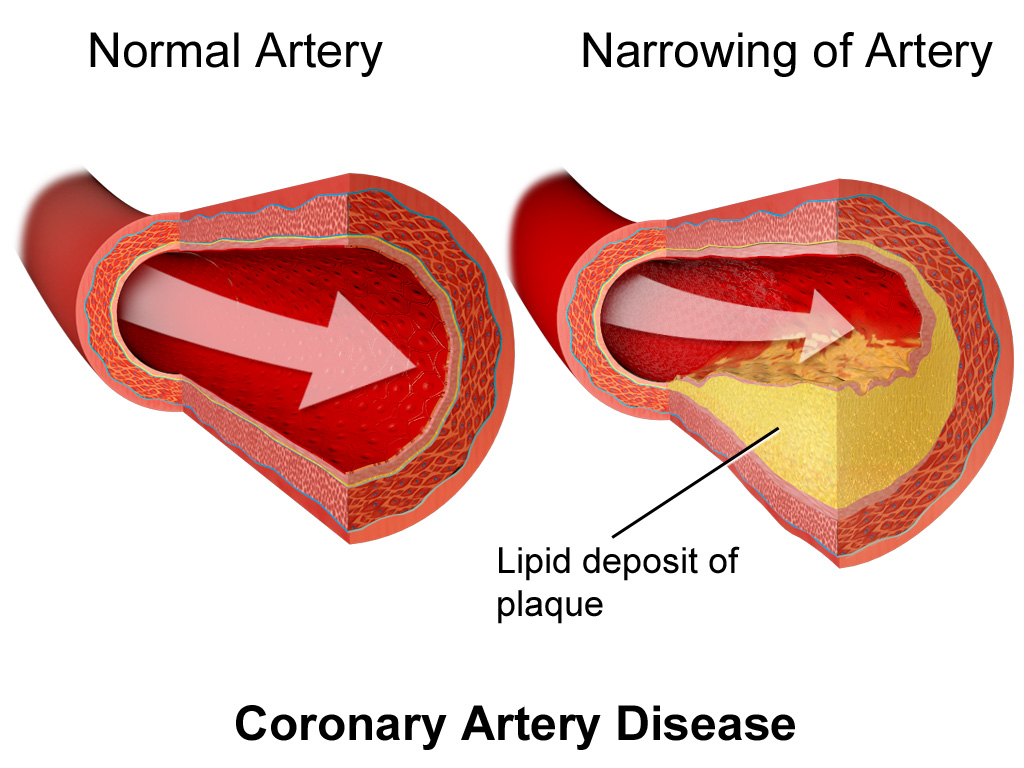
The care of patients with coronary artery disease is very complex, and there is enormous variability between different healthcare centres. The most recent consensus guidelines recommend a CT scan as a first-line test for these patients. This project wants to go further and not only prove the diagnostic capabilities of the CT scan, but also show its usefulness for patient risk stratification, to obtain additional insights and plan the intervention, and to follow-up the patients afterwards.
For these goals, the project will make use of new technologies that are available to us. In first place, a new generation of CT machines that offer additional capabilities, such as the spectral CT and the photon-counting CT, that offer increased resolution and additional information respect to older generations of CT machines. In second place, the use of AI technologies to improve the image quality of the scan, and to combine all available information to provide new insights. Finally, integration of the CT scan information at all steps of the workflow, in particular in the intervention room, will provide an opportunity to use the CT in ways that were not used before.
To prove that all our aims are possible, we have set up 5 clinical trials in which we will test the developments of the project. These are the CODEX1, CONVENE, EVOLVE, IMPACT and SPCCT-STENT trials, which will cover the different steps of the care of patients with coronary artery diseases, and with which we hope to show all the advantages of using a CT based for workflow for this disease.
All in all, this project will deliver futureproof cardiology care for our patients.
Our Clinical Trials

The first step in the care of patients with suspected coronary artery disease workflow is to have an accurate diagnosis. The COMBINE-CT project aims to offer a diagnostic application with a highly intuitive workflow and automated detection of coronary lesions, based on images of the CT-scan. The CODEX1 trial will gather images and data from patients that had both a CT-scan and second test that detects ischaemia (lack of oxygen to the heart due to a coronary lesion) or an interventional procedure that offers a definitive diagnosis, and will compare the results from our diagnostic application with those of the second test.

The CT scan can be a very useful tool to additional insights and plan percutaneous coronary intervention. For that reason, the combine-CT project will run the CONVENE trial, which will measure the benefits of using a CT-scan before a percutaneous coronary intervention, in patients with stable coronary disease.

The follow-up of patients with high risk to develop coronary artery disease is also a challenge that the combine-CT project wants to address throughout the use of the CT scan. In this trial, we will focus on type II diabetes mellitus patients, which is an important risk factor to develop coronary artery disease, and who are patients which will benefit of having their treatment optimized as the disease progresses. For that reason, we will use the CT scan to perform anatomical evaluation in these patients at set intervals and evaluate the feasibility of quantifying the disease progression.

The best way to get information about a coronary artery lesion is by means of invasive percutaneous techniques, such as intracoronary imaging, or the invasive measurement of blood pressure and blood flow inside the coronary artery. These techniques are not always used during an intervention, but are required in some cases such as characterizing a high-risk lesion before to choose the best intervention technique, or deciding if a moderate-looking lesion is severe enough to warrant a stent implant.
With the IMPACT trial, we want to get as close to this information while using a non-invasive CT scan. For that, we will gather data from patients with both invasive information and a CT scan, and use artificial intelligence techniques to increase the diagnostic accuracy of the CT for determining the severity of the coronary artery lesions.

Our final clinical trial deals with patients who have already been treated for coronary artery disease and have had a stent implanted. Evaluating the status of an implanted stent is a difficult task for a traditional CT machine, but new CT technology, such as spectral photon counting CT, results in a higher resolution image that could be used for this purpose. In this trial, we will study patients who underwent a high-risk percutaneous artery intervention and will be followed-up for stent patency evaluation.
Video
Our Partners
In this newsletter we will introduce two of them:

The Instituto de Investigación Biomédica de Salamanca (IBSAL) is an entity created on 2011, through an agreement between the regional government of Castilla y León and the University of Salamanca, with the incorporation of the Spanish National Research Council (CSIC) in 2012. This institute is dedicated to biomedical research, encompassing basic, clinical, epidemiological, and health services research. IBSAL coordinates and promotes biosanitary research activities across various institutions, including the University Hospital of Salamanca, the Primary Care Management of Salamanca, and the biosanitary areas of the University of Salamanca, including the Institute of Neurosciences of Castilla y León and the Institute of Molecular and Cellular Biology of Cancer. IBSAL’s mission is to strengthen translational research by fostering collaboration between clinical and basic research groups to optimize resources and promote synergies. Its vision is to establish itself as a reference institute in Castilla y León and northwest Portugal, with a particular focus on areas such as cancer, neurosciences, and cardiovascular research. With a commitment to innovation and excellence, IBSAL aims to be a bridge between scientific knowledge and its practical application, driving the development of the biosanitary campus and facilitating the transfer of knowledge to clinical practice.
IBSAL is involved in all the work packages of the project, and leads tasks such as the CODEX1 clinical study, or an AI-based predictive model to improve diagnostic performance of the CT scan.

The Centro de Investigación Biomédica En Red (CIBER) is a public consortium created in 2006 by the Carlos III Health Institute (ISCIII) with the aim of promoting excellence in biomedical and health sciences research in Spain. CIBER supports research through networks of knowledge formed by research centers and groups from various public and private administrations and institutions. This networked research model facilitates collaboration to improve the efficiency and effectiveness of projects. Currently, CIBER has 831 employees and over 5,600 researchers distributed across 518 research groups that collaborate with 104 partner institutions in various autonomous communities of Spain. This collaborative approach allows CIBER to reduce the time needed to transfer its findings to the National Health System, thereby enhancing the quality of life for citizens and promoting social well-being.
CIBER is the leading partner in the work package “Dissemination, Communication and Exploitation” with a small role in other work packages.
The Consortium











